Ocrelizumab, serum immunoglobulin levels and serious infections
SI and IgG analyses are presented for the Phase III OPERA (RMS) and ORATORIO (PMS) populations only
- After 11 years of continuous OCR treatment, IgG levels remained above the LLN for >80% of patients with RMS and PPMS
- During episodes of IgG<LLN, the type, severity and outcome of SIs were consistent with the overall SIs observed in patients treated with ocrelizumab during the controlled period and OLE phase
Clinical trial population: Relationship between SI rates and Ig levels3
COVID-19 related AEs were excluded from this analysis, but patients continued to contribute to the incidence of all other AEs
- Based on a multivariate analysis, there is a weak association between IgG and risk of SIs
- Other risk factors include comorbidities, disability progression, on-study relapses, increased BMI and abnormal IgM levels
SIs associated with low IgG levels by MS type: OPERA (RMS) and ORATORIO (PPMS)2
- During a period with IgG<LLN, 2.0% of patients with RMS and 1.9% of patients with PPMS had an SI
- UTIs, LRTIs, sepsis, abdominal and GI infections, and skin infections were the most common SIs associated with episodes of IgG<LLN, a pattern similar to overall SIs
Figure 1: IgG<LLN and SIs: Outcome and action taken with OCR2
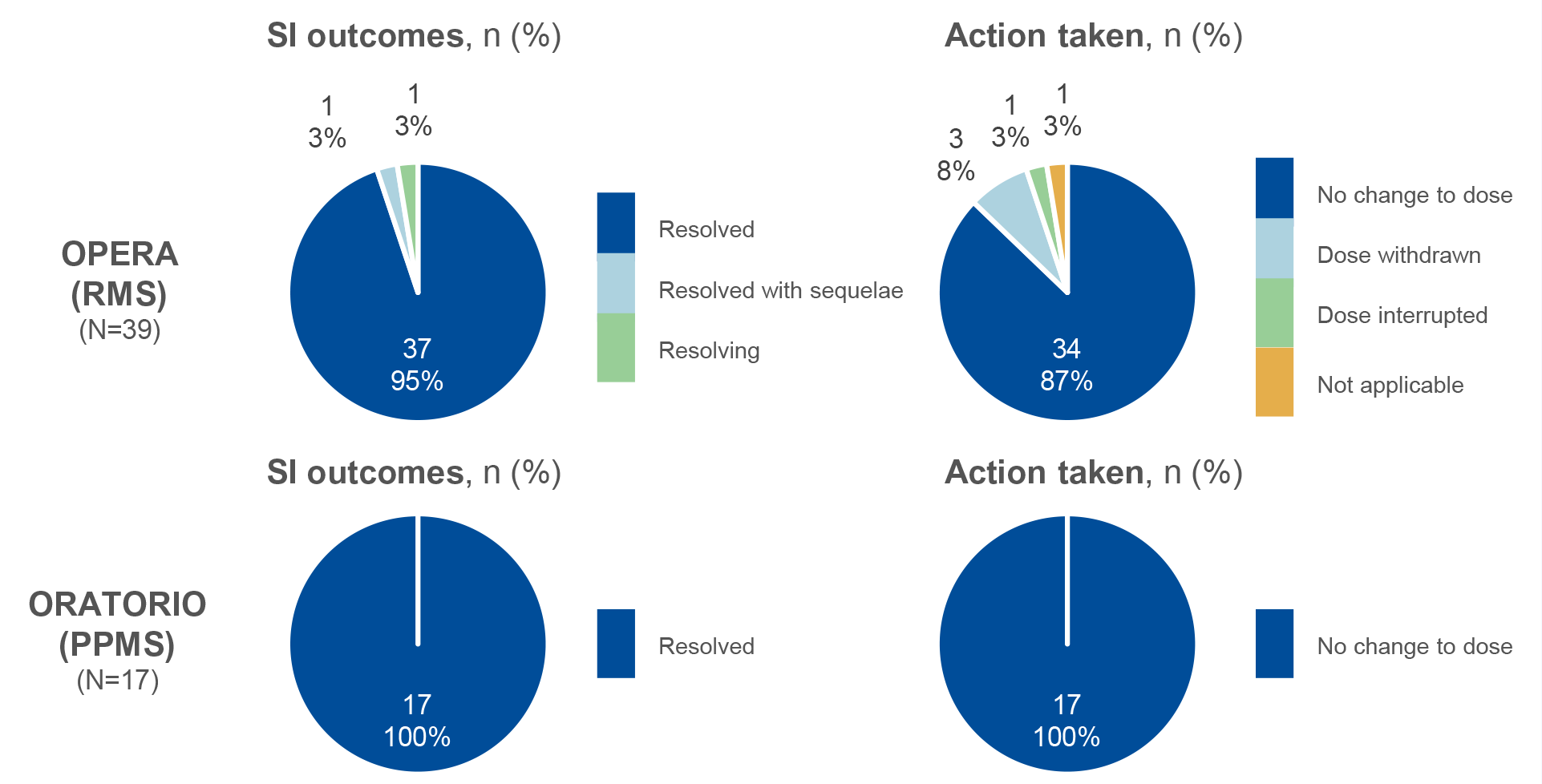
- During periods of IgG<LLN, there were no fatal events, most SIs resolved and treatment with OCR was continued in almost all cases
For more information on the rate of infections with OCR treatment, please visit the Ocrelizumab and infections
Figure 1: n indicates the number of events. Percentages may not total 100% due to rounding. SI and IgG analyses are presented for the Phase III OPERA (RMS) and ORATORIO (PPMS) populations only. Abbreviations: AE, adverse event; BMI, body mass index; COVID-19, coronavirus disease 2019; Ig, immunoglobulin; GI, gastrointestinal; LLN, lower limit of normal; LRTI, lower respiratory tract infection; OCR, ocrelizumab; OLE, open-label extension; PPMS, primary progressive MS; RMS, relapsing MS; SI, serious infection; UTI, urinary tract infection.
Indications vary in different countries. The local prescribing information from your country is the primary source of information on the known and potential risks associated with ocrelizumab.
References
- Hauser SL, et al. Presented at ECTRIMS 2023 (Poster P304);
- Hauser SL, et al. Presented at ECTRIMS 2024 (Poster P300);
- Derfuss T, et al. Ther Adv Med Oncol 2024;17:10.1177/17562864241277736.
M-XX-00019233 (Date of preparation: December 2024)