Ocrelizumab and infections
- PwMS are at greater risk of developing, and being hospitalised for, infections than the general population1–3

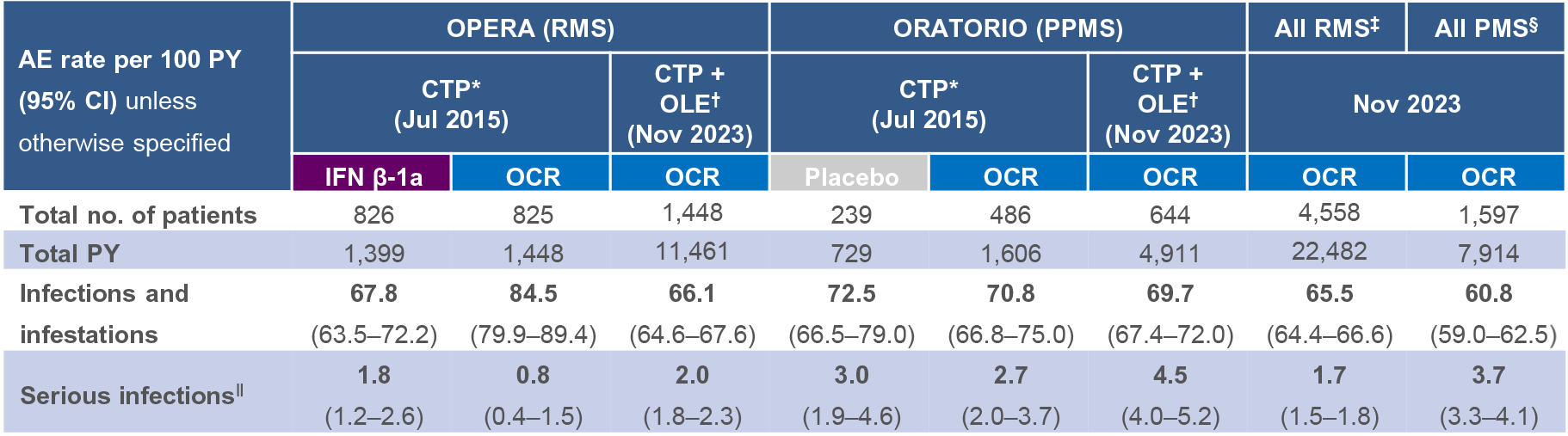
- In OCR RMS and PPMS pivotal clinical trials, infections were a frequently reported AE4–7
- However, no increased risk of serious infections (SIs) with OCR vs IFN β-1a or placebo was observed during the controlled treatment period4–7
- During the open-label extension phase in patients with RMS and PPMS, the overall risk of SIs did not increase from that observed during the controlled treatment period6,7

- Over an 11-year follow-up period in clinical trials*, OCR continues to exhibit a stable and favourable safety profile6
Clinical trials (controlled treatment period and open-label extension)
Incidence of infections in OCR clinical trials per 100 PY6
Table 1: OPERA (RMS), ORATORIO (PPMS), all RMS and all PMS cumulative exposure (controlled treatment period and open-label extension)
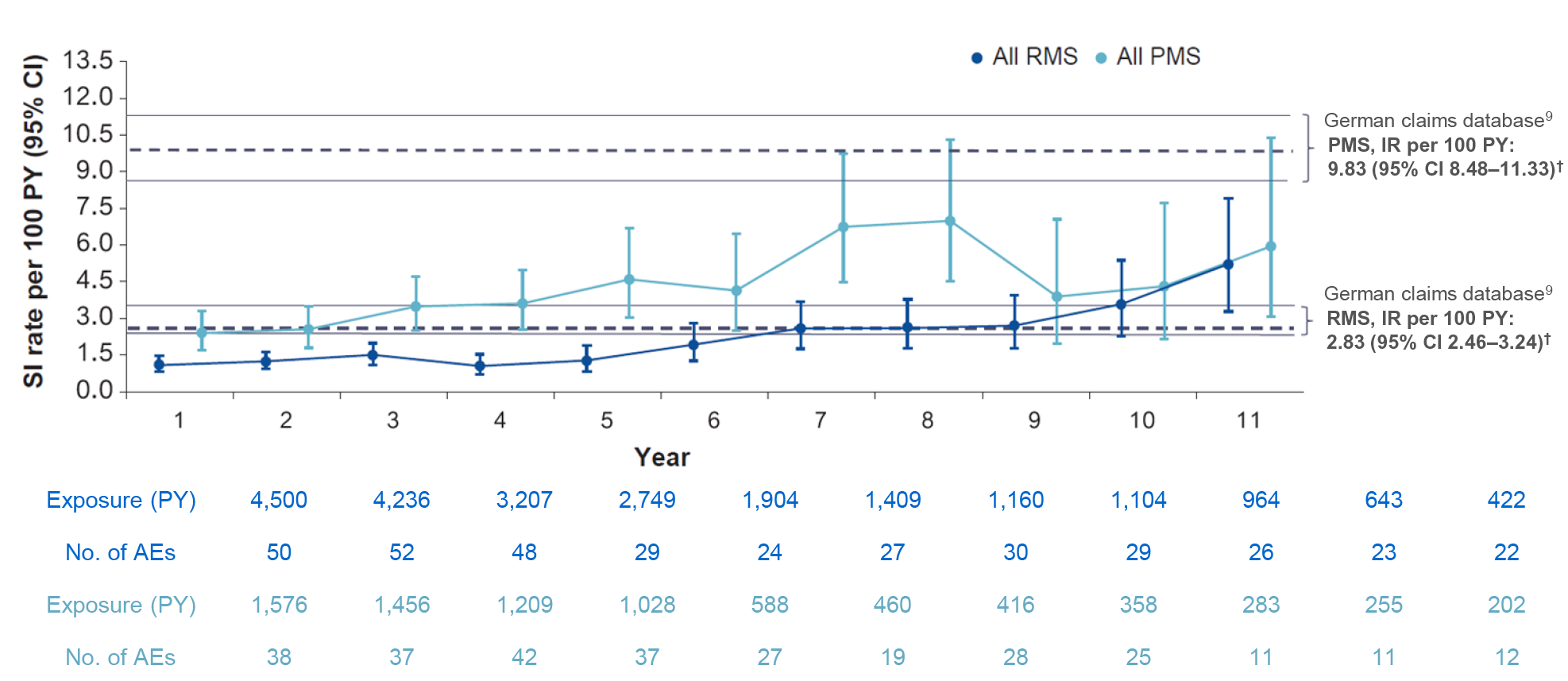
- In PPMS, the rate of SIs remained higher than RMS;6 over time, this could be due to the underlying disease condition (e.g. increasing disability, age, comorbidities)8
Figure 1: Yearly rate of SIs (excluding COVID-19*) in RMS and PMS all-exposure populations6
Clinical cut-off date: November 2023
- The majority of SIs (>88%) resolved without sequelae and within ≤2 weeks, and most patients remained on treatment with OCR6
- In the RMS and PMS all-exposure populations, LRTIs (mostly pneumonia) and UTIs were the most commonly reported types of SIs; this is consistent with incidence rates and patterns observed in real-world studies1,2,6,9,10
- SIs remained infrequent with rates consistent with ranges in real-world studies1,6,9
Figure 2: Multivariate model for risk of SIs in OPERA (RMS)11
Clinical cut-off date: December 2022
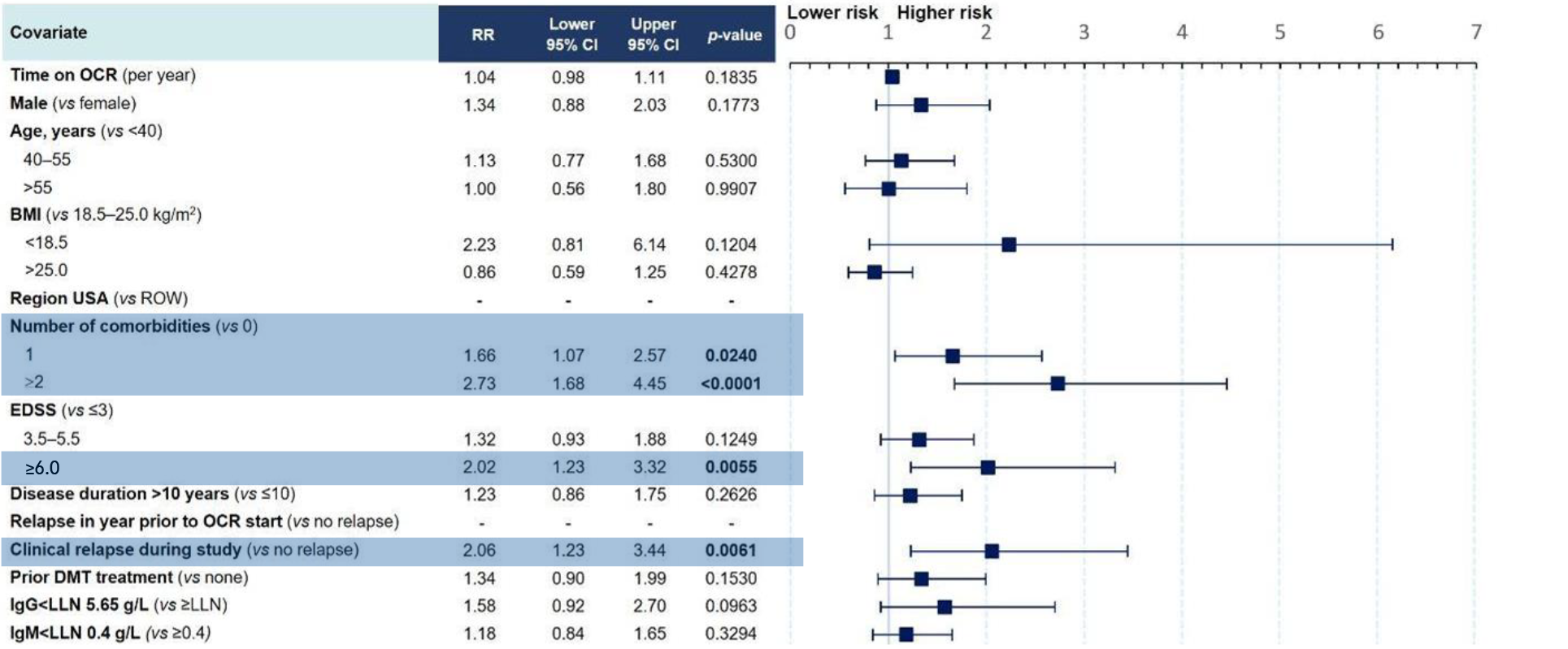
Figure 3: Multivariate model for risk of SIs in ORATORIO (PPMS)11
Clinical cut-off date: December 2022
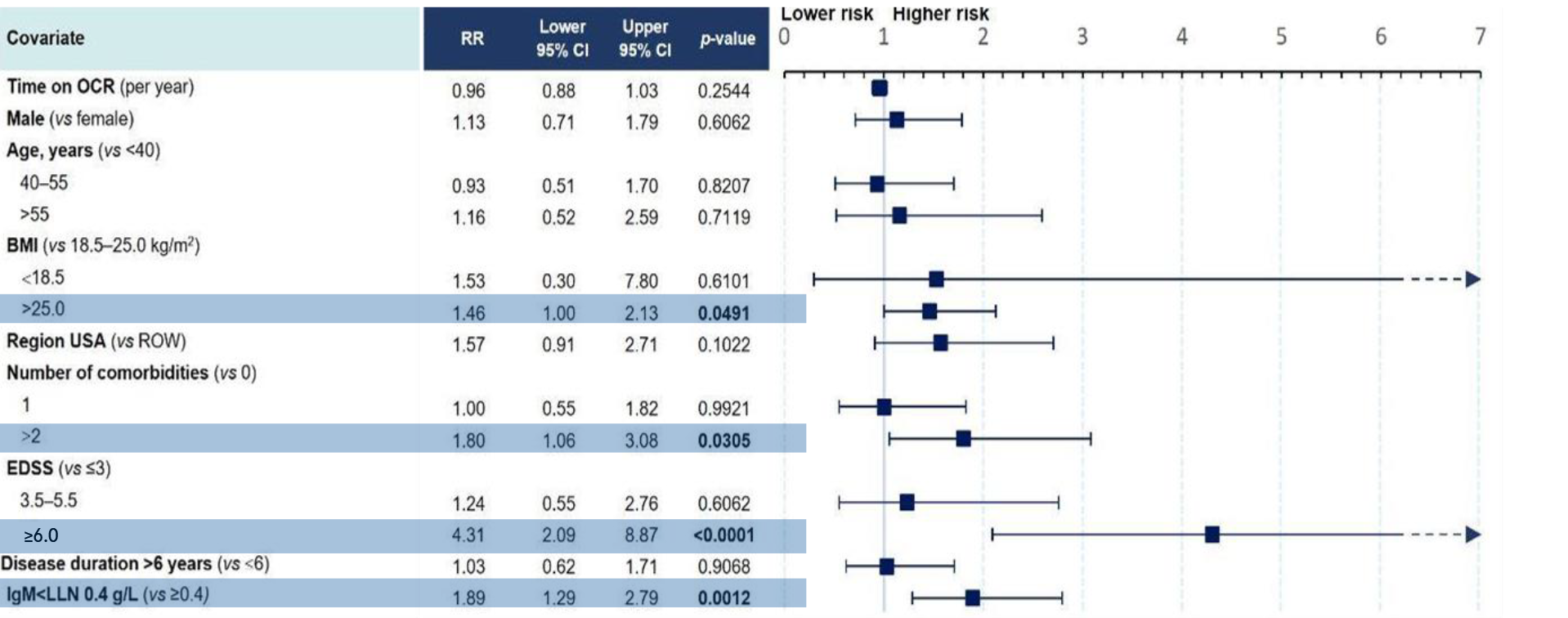
- Time on treatment with OCR and decreased IgG levels were not associated with an increased risk of SIs11
- The presence of comorbidities, having an EDSS ≥6.0 and experiencing study relapses were associated with an increased risk of SIs in people with RMS11
- Being overweight or obese, having ≥2 comorbidities, having an EDSS ≥6.0, and having abnormal IgM levels were found to be associated with an increased risk of SIs in people with PPMS11
For more information
on IgG levels and SIs
with OCR, please
visit the Ocrelizumab
and serum IgG levels webpage
As of March 2024:
- Over 350,000 patients with MS have started OCR in post-marketing and clinical trial settings globally, corresponding to an exposure of >1,000,000 PY12
- No new findings related to the type or pattern of SIs were identified12
- In these post-marketing case reports, the most commonly reported SIs by preferred terms, excluding COVID-19, were UTI and pneumonia, which is in line with clinical trial data12
Overview: *Includes patients who received any dose of OCR during the CTP and associated OLE periods of the Phase II (NCT00676715) and Phase III (NCT01247324, NCT01412333, NCT01194570) studies plus VELOCE (NCT02545868), CHORDS (NCT02637856), CASTING (NCT02861014), OBOE (NCT02688985), ENSEMBLE (NCT03085810), LIBERTO (NCT03599245), CONSONANCE (NCT03523858), CHIMES (NCT04377555) and OLERO (NCT05269004), including patients originally randomised to comparator (IFN β-1a or placebo) who switched to open-label OCR treatment (data as of November 2023). Table 1: COVID-19 related AEs were excluded from this analysis, but patients continued to contribute to the incidence of all other AEs. AEs were classified according to MedDRA versions 18.0, 18.1, 22.1 and 24.1. Multiple occurrences of the same AE in one patient are counted multiple times. *Data as of April–July 2015; †Includes patients who received any dose of OCR during the CTP and associated OLE periods of the Phase III studies, including patients originally randomised to comparator (IFN β-1a or placebo) who switched to open-label OCR treatment (data as of November 2023); ‡Includes patients with RMS who received any dose of OCR during the CTP and associated OLE periods of the Phase II (NCT00676715) and Phase III (NCT01247324, NCT01412333) studies plus VELOCE (NCT02545868), CHORDS (NCT02637856), CASTING (NCT02861014), OBOE (NCT02688985), ENSEMBLE (NCT03085810), LIBERTO (NCT03599245), CHIMES (NCT04377555) and OLERO (NCT05269004). Data as of November 2023; §Includes patients with PMS who received any dose of OCR during the CTP and associated OLE periods of OBOE (NCT02688985), ORATORIO (NCT01194570), CONSONANCE (NCT03523858) and OLERO (NCT05269004). Data as of November 2023; ‖SIs are defined using AEs falling into the MedDRA SOC ‘Infections and Infestations’, and using ‘Is the event nonserious or serious?’ from the AE case report form. Figure 1: COVID-19 related AEs were excluded from this analysis, but patients continued to contribute to the incidence of all other AEs. *More information on COVID-19 related SI rates can be found in the supplementary material; †Cumulative rates of SIs over a median period of 3.9 years (2016–2019) in a German claims database.9 For comparability, the rates were calculated in a subpopulation of patients with RMS and PMS up to the age of 64 years. Post-marketing: *There are well-recognised limitations that should be considered when interpreting spontaneous post-marketing safety reports, including events that may not be causally related to drug exposure; in the real-world setting, events are frequently confounded by factors such as multiple drug use and the presence of pre-existing comorbidities; reporting bias may exist for more significant outcomes, which may result in an overrepresentation of the more serious outcomes; and reporting rates can be stimulated by external factors, such as press reports. The causes of infections are recorded as reported to the company; while the company follows up on all reports to identify the cause, an exact diagnosis is not always possible. Some of the investigations remain ongoing and, therefore, the information may be subject to change. Abbreviations: AE, adverse event; BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; CTP, controlled treatment period; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; IFN β-1a, interferon beta-1a; Ig, immunoglobulin; IR, incidence rate; LLN, lower limit of normal; LRTI, lower respiratory tract infection; MedDRA, Medical Dictionary for Regulatory Activities; OCR, ocrelizumab; OLE, open-label extension; PMS, progressive MS; PPMS, primary progressive MS; PY, patient-years; pwMS, people with MS; RMS, relapsing MS; ROW, rest of world; RR, rate ratio; SI, serious infection; SOC, System Organ Class; UTI, urinary tract infection.
Indications vary in different countries. The local prescribing information from your country is the primary source of information on the known and potential risks associated with ocrelizumab.
References
- Wijnands JMA, et al. Mult Scler 2017;23:1506–16;
- Nelson RE, et al. Int J MS Care 2015;17:221–30;
- Wijnands JMA, et al. J Neurol Neurosurg Psychiatry 2018;89:1050–6;
- Hauser SL, et al. N Engl J Med 2017:376:221–34;
- Montalban X, et al. N Engl J Med 2017;376:209–20;
- Hauser SL, et al. Presented at ECTRIMS 2024 (Poster P300);
- OCREVUS [SmPC]. https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf. Accessed 28 November, 2024;
- Hauser SL, et al. Presented at ECTRIMS 2022 (Poster P326);
- Knapp R, et al. Mult Scler Relat Disord 2022;68:104245;
- Persson R, et al. Mult Scler Relat Disord 2020;41:101982;
- Derfuss T, et al. Ther Adv Neurol Disord 2024;17:17562864241277736;
- Roche data on file.
M-XX-00019237 (Date of preparation: December 2024)